Background
Central nervous system (CNS) recurrence of systemic diffuse large B cell lymphoma (DLBCL) is a well-recognized and almost uniformly fatal complication that tends to occur within 2 years of initiating treatment. The probability of CNS relapse in DLBCL ranges from 4 to 7% overall but is increased to 20% in certain subgroups. Evidence supporting the use of intravenous methotrexate (MTX) or arabinoside (AraC) in high-risk patients is growing, however, the use of other agents remains to be defined. Thiotepa (TT) is a polyfunctional alkylating agent first developed in the 1950s. Both thiotepa and its metabolite tepa demonstrate good CNS bioavailability, making thiotepa a useful adjunctive chemotherapy for primary and secondary CNS lymphoma, particularly in the context of hematopoietic stem cell transplant (HSCT). Herein, we conduct this prospective trial to evaluate the efficacy and safety of prophylactic intrathecal thiotepa for the prevention of secondary CNS involvement in patients with DLBCL.
Methods
Patients
The primary end point is 2-year cumulative incidence of CNS. The secondary end points are 2-year progression free survival (PFS), 2-year overall survival (OS), and safety.
The main inclusion criteria include: adults patients ≥18 years old; histopathological confirmed DLBCL without previous antitumor therapy; high-risk CNS involvement (one of the following): primary breast or testicular lymphoma, involvement of any of the following organs: testis, breast, kidney, adrenal gland, paranasal sinus, epidural space or uterus; CNS International Prognostic Index (CNS-IPI)≥4; dual protein expression (DE) or double/triple hit (DH/TH) lymphoma.
The main exclusion criteria include: Burkitt lymphoma, primary CNS lymphoma, or large B cell lymphoma transformed from indolent lymphoma; the presence of parenchymal or meningeal lymphoma; history of whole brain radiotherapy or craniospinal cord irradiation; obstructive hydrocephalus patients requiring neurosurgical intervention.
Treatment protocol
The systemic chemotherapy regimen is determined by investigator. All patients are need to receive IT thiotepa 10 mg and dexamethasone 5 mg on day 1 of each cycle of chemotherapy for at least 4 cycles. All patients are asked to lie flat for 4 to 6 hours after the lumbar puncture.
Follow-up
Routine biochemical and cytological analysis of cerebrospinal fluid is performed for each cycle. Imaging examination, such as brain magnetic resonance imaging (MRI) and body computed tomography (CT), is repeated every 2 cycles of chemotherapy, then every 3 months during follow-up.
Results
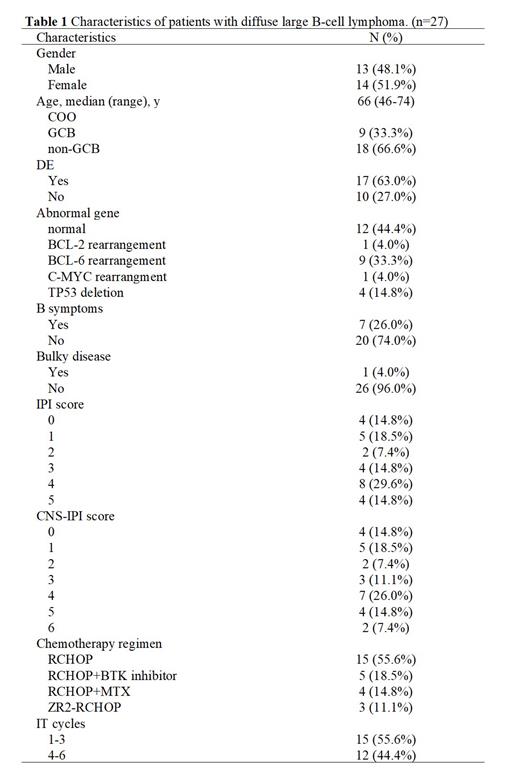
From February 2022 to June, 2023, 27 patients (female 14, male 13) received IT thiotepa. The clinical characteristics are shown in Table 1. The median age was 66 years old (range: 46-74). As first-line chemotherapy, 15 (55.6%) patients received rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (RCHOP), 5 (18.5%) patients received RCHOP+BTK inhibitor, 3 (11.1%) patients received BTK inhibitor, lenalidomide with rituximab (ZR2) followed by RCHOP, and 4 (14.8%) patients received RCHOP with intravenous MTX. Thirteen (48.1%) patients had IPI score 4-6 and 17 (63%) patients had DE of c-myc and bcl-2. All patients received fluorescence in situ hybridization (FISH) cytogenetic testing for C-MYC, BCL-2, BCL-6 and TP53. Four (14.8%) patients had TP53 deletion, and 11 (40.7%) patients had sole rearrangement of C-MYC or BCL-2 or BCL-6, but no one were diagnosed with DH/TH lymphoma. All patient have received IT thiotepa for at least once, and 12 (44.4%) patients completed 4-6 cycles of IT, 84 cycles totally. IT thiotepa was a safe measure, for no one experienced post-LP headache, local infection, bleeding, or cerebral herniation. But there were 4 patients had five times of inability to identify direction and slow speech response. They could recover normally the next day without any treatment. Although the follow-up period was short, no patient developed CNS involvement.
Conclusion
Prophylactic intrathecal thiotepa is an effective and side-effects manageable measure for preventing secondary central nervous system involvement in high-risk DLBCL. Longer follow-up and larger-scale prospective trial is needed to testify this measure.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal